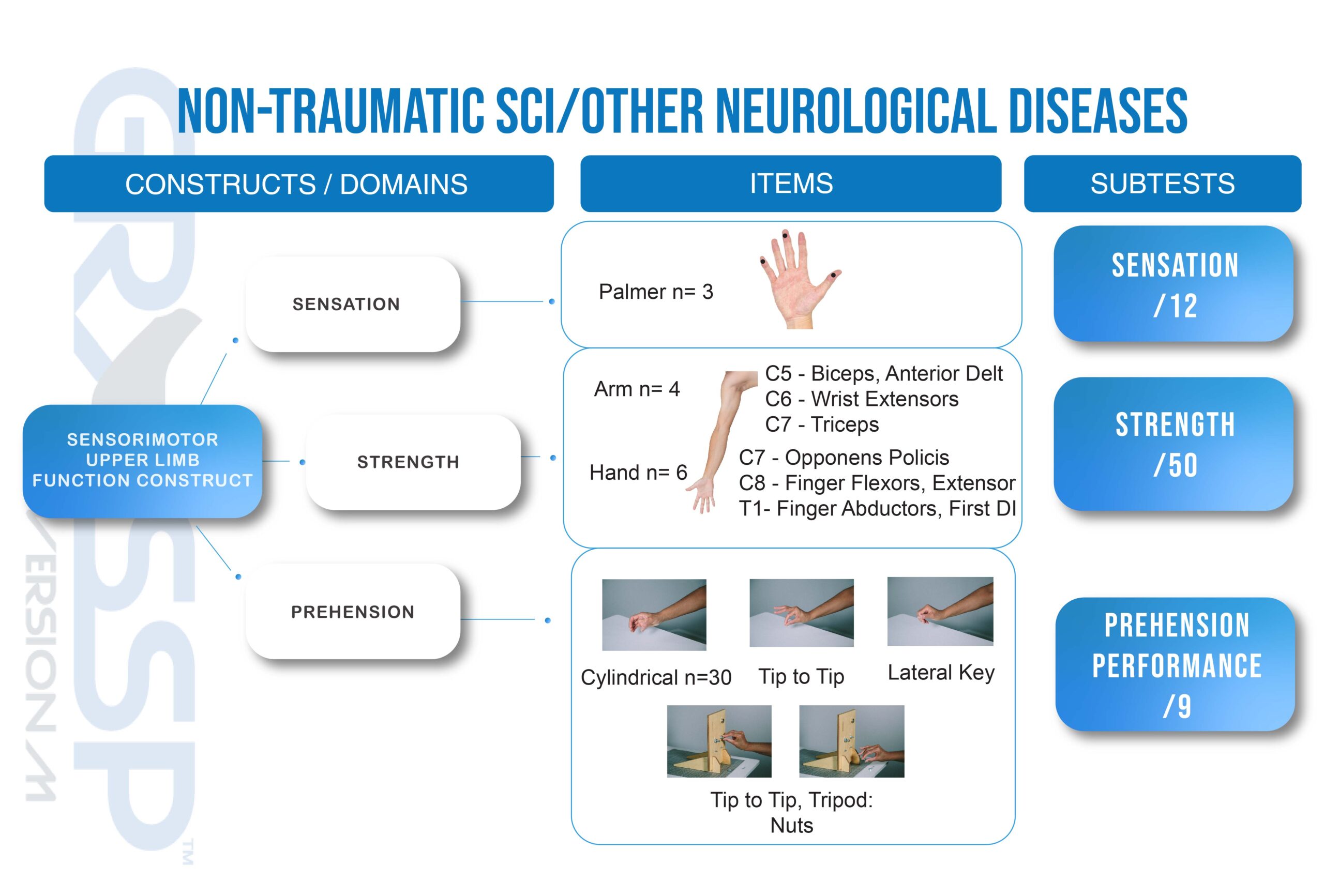
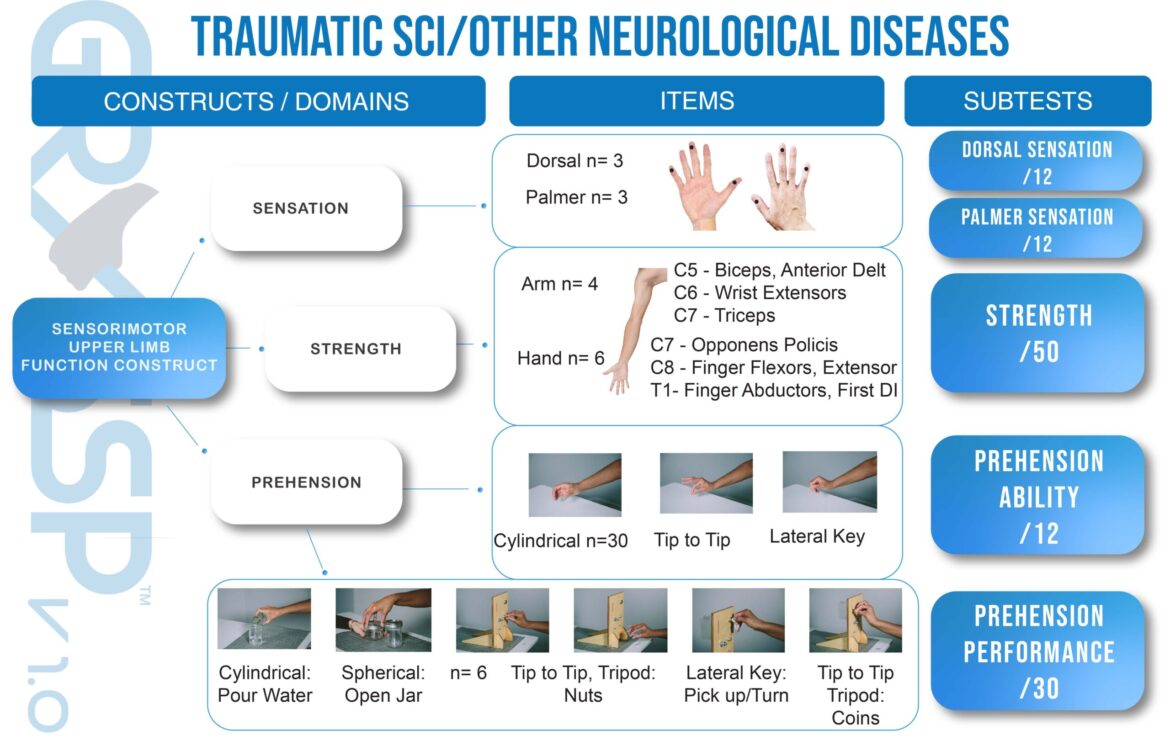
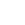ORIGIN OF GRASSP
GRASSP Version 1 (GV1) was developed in 2006 by the GRASSP Development and Research Group. It was designed using elements of the Link Hand Function Test, Quadriplegia Hand Assessment Tool and Sollerman Hand Function Test. GV1 has been tested for the psychometric properties during early development. The psychometric properties are excellent; evidence for the reliability, validity and responsiveness are published.
Development of the GRASSP has been a staged process with a number or different research groups and funding agencies involved. Once the GRASSP V1 had been assembled and GRDG conducted a cross sectional study to field test and establish the psychometric properties of reliability and validity.
CROSS SECTIONAL STUDY
This study was conducted in Europe, Canada and the USA. Seventy-two patients were enrolled into a study designed to evaluate the reliability and validity of the GRASSP. This study was conducted on a sample of chronic patients.
Cross Sectional Study Team Investigators:Sukhvinder Kalsi-Ryan
Professor Molly Verrier
Dr. Michael Fehlings
Dr. Armin Curt
Dr. Dorcas Beaton
Dr. Milos Popovic
Claudia Rudhe
Susan Duff.
Balgrist University Hospital Zurich Switzerland
| Claudia Rudhe | GRDG, Examiner |
| Christina Robert | Examiner |
Krakenhaus Hohe Worte Bayreuth Germany
| Kristin Lorenz | Examiner |
| Petra Schatz | Examiner |
Traumacenter Murnau, Murnau Germany
| Martha Horn | Examiner |
| Simone Hirsch | Examiner |
Rehabilitation Institute of Chicago USA
| Kimberley Eberhardt | Examiner |
| Rebecca Ozelie | Examiner |
Thomas Jefferson University Philadelphia USA
| Gina Cooke | Examiner |
| Susan Duff | Examiner |
Vancouver Coastal Health Vancouver, Canada
| Dr. Armin Curt | GRDG |
| Megan Watts | Examiner |
| Robert Corcoran | Examiner |
Toronto Rehab Institute, Toronto Canada
| Sukhvinder Kalsi-Ryan | GRDG, Examiner |
| Marlene Adams | Examiner |
| Sylvia Coates | Examiner |
| Abigail Dry | Examiner |
| Professor Molly Verrier | GRDG |
UHN, Toronto Canada
| Dr. Michael Fehlings | GRDG |
Longitudinal Study Group
This study was a longitudinal study enrolling 130 patients in Canada and Europe to establish responsiveness, recovery profiles and minimally clinical important difference (MCID). The responsiveness of the GRASSP is excellent and it is more sensitive than some of the existing measures in SCI. Data collection for the longitudinal study concluded in November of 2014. MCID has been established, but not yet published. This longitudinal dataset has also enable the team to perform Rasch Analysis, which has rendered a revision to GRASSP Version 1. The GRDG is currently in the process of launching GRASSP Version 2.
Investigators:
| Sukhvinder Kalsi-Ryan | Dr. Dorcas Beaton |
| Professor Molly Verrier | Dr. Milos Popovic |
| Dr. Michael Fehlings | Dr. Inge Marie Velstra |
| Dr. Armin Curt |
Canadian GRASSP Longitudinal Study Group
Toronto Rehab, Toronto
| Professor Molly Verrier | Site Investigator |
| Vera Zivanovic | Research Coordinator, Examiner |
| Naz Kapadia | Examiner |
Toronto Western Hospital
| Sukhvinder Kalsi-Ryan | Site Investigator |
| Jean Brown | Examiner |
| Kathy Murphy | Examiner |
St. Michael’s Hospital
| Dr. Henry Ahn | Site Investigator |
| Kayee Tung | Research Coordinator |
| Faye Uy | Examiner |
| Zia Poonjiaji | Examiner |
| Kerry Doherty | Examiner |
Sunnybrook Health Sciences Centre
| Dr. Michael Ford | Site Investigator |
| Aimee Gallant | Research Coordinator |
| Jocelyn Denomme | Examiner |
Hamilton Health Sciences Centre
| Dr. Brian Drew | Site Investigator |
| Deborah Tsui | Research Coordinator |
| Sera Nicosia | Examiner |
| Barbara Murray | Examiner |
| Dorinda Taylor | Examiner |
| Mirela Anton | Examiner |
| Dee Whitman | Examiner |
London Acute (St.Joseph's)
| Dr. Chris Bailey | Jennifer Fleming |
| Jennifer Fleming | Research Coordinator, Examiner |
London Parkwood Rehab
| Dalton Wolfe | Site Investigator |
| Heather Askes | Research Coordinator |
| Sarah Miles | Examiner/Investigator |
| Karen Gilbert | Examiner |
| Andy Morton | Examiner |
| Ange Woolner | Examiner |
European GRASSP Longitudinal Study Group
Balgrist, University Hospital, Zurich
| Marc Bolliger/Armin Curt | Site Investigator |
| Urs Albisser | Examiner (Site Coordinator) |
| Hartmut Pöhlmann | Examiner |
| Anja Bodenmann | Examiner |
| Josch Wagner | Examiner |
| Karin Akermann | Examiner |
| Claudia Rudhe | Examiner |
Swiss Paraplegic Center Nottwil, Nottwil
| Inge-Marie Velstra | Research Coordinator, Examiner |
| Diana Sigrist Nix | Examiner |
| Emanuela Albisetti | Examiner |
| Friederike Ebert | Examiner |
| Bart de Kimpe | Examiner |
BG-Kliniken Bergmannstrost, Halle
| Daniel Kuhn | Examiner (Site Coordinator) |
| Kerstin Rolle | Examiner |
| Diana Pannier | Examiner |
| Reinhard Keck | Examiner |
| Carola Losel | Examiner |
Berufsgenossenschaftliche Unfallklinik Murnau, Murnau
| Denise Haupt | Examiner (Site Coordinator) |
| Maren Bauch | Examiner |
Krankenhaus Hohe Warte Bayreuth, Bayreuth
| Kristin Lorenz | Examer/Site Coordinator |
| Petra Schatz | Examiner |
| Elke Engelbrecht | Examiner |
Stiftung Orthopädische Universitätsklinik Heidelberg
| Ruth Krahl | Examiner (Site Coordinator) |
| Marlis Euler | Examiner |
Zentrum für Rückenmarkverletzte Werner-Wicker Klinik Bad Wildungen, Bad Wildungen-Reinhardshausen
| Uta Mesecke | Examiner (Site Coordinator) |
| Fr.M.Wagener | Examiner |
| Fr.S.Eichenberg | Examiner |